Photocatalysis on metal oxide surfaces
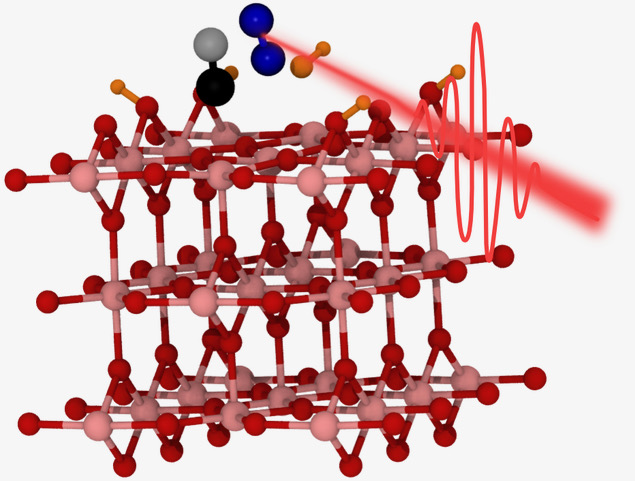
Femtosecond X-ray laser pulses synchronized with an optical laser were employed to investigate the dynamics of the photocatalytic oxidation of CO on the anatase TiO2(101) surface in real-time. Our results provide evidence of ultrafast timescales for the photooxidation of CO to CO2 and clarify the initial mechanism of oxygen activation that is crucial to unraveling the underlying processes for a range of photocatalytic reactions relevant to air purification and self-cleaning surfaces. The photocatalytic oxidation of CO takes place between 1.2 - 2.8 ( 0.2) ps after irradiation with an ultrashort laser pulse leading to the formation of CO2, prior to which no intermediate species were observed. We found that the presence of intragap unoccupied O2 levels leads to the formation of a charge transfer complex that can be activated by visible light at a photon energy of 1.6 eV, in line with theoretical calculations. This allows the reaction to be initiated via a newly proposed mechanism involving the direct transfer of electrons from TiO2 to physisorbed O2.